The preparation of pharmaceutical tablet is based on the judicious choice of the ingredients and their owners: the drug substance, the excipients, the auxiliary substances and the packaging containers, as well as the pharmaceutical operations constituted in a technological stream. The formulation of the drug and the manufacturing process must ensure the desired quality of the pharmaceutical for mand its reproducibility under the conditions of the industrial preparation. The rational choice of the physicochemical and biopharmaceutical properties of the drug and the excipients as well as the stages of the manufacturing process are only the qualitative element of the formulation.
Pharmaceutical Drug Formulation
Each pharmaceutical formulation of a drug is unique in its biopharmaceutical behavior, so it can potentially be an important determinant factor of the efficacy and safety of the clinical response. Most of the pharmaceutical preparations that are currently being used are composed of solid dosage forms and constitute the pharmaceutical form more used, which represents between 40 and 70 % of the total production of the pharmaceutical forms commercially available. In these pharmaceutical forms, the amount of drug that reaches the site of action depends on the release of this from the pharmaceutical form.
Solid Pharmaceutical Tablets
Solid tablets are commonly used in various applications such as medicine, agriculture, or water purification. In the pharmaceutical industry, solid tablets are one of the most popular dosage forms. The reason behind this is that tablets are relatively easy to manufacture, store and transport, and provide a convenient way to administer drugs. The majority of the pharmaceutical tablets are coated as a part of the manufacturing process. The coating serves several functions such as odour and taste masking, increasing swallowability, physical and chemical protection during storage and transportation, increase aesthetic appeal and control drug release profile.
.

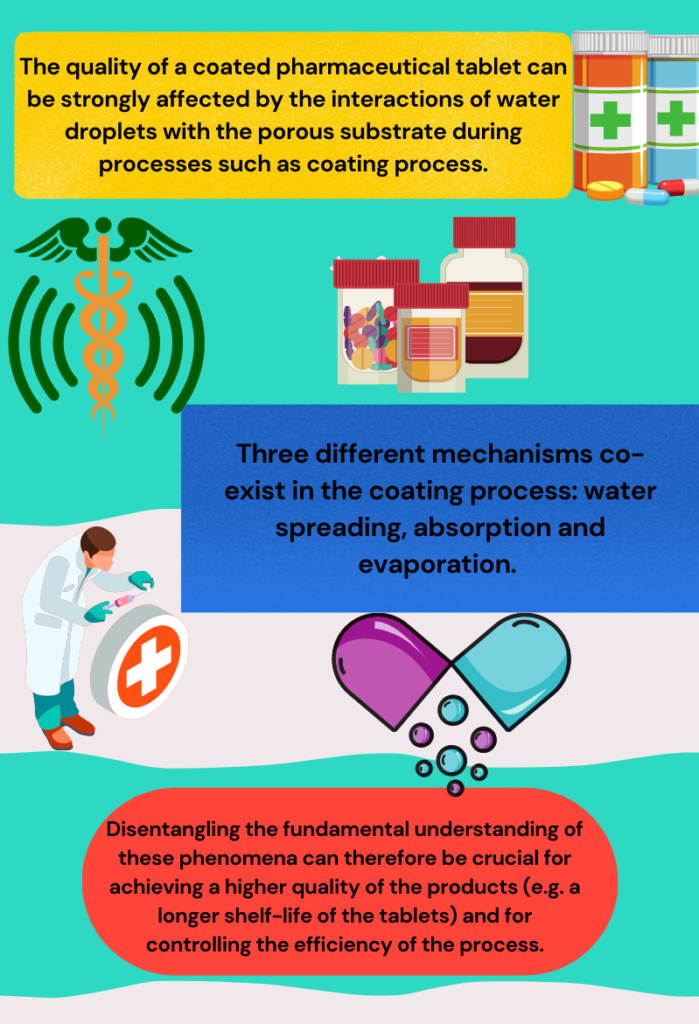
Water transport and absorption in pharmaceutical tablets
The quality of a coated pharmaceutical tablet can be strongly affected by the interactions of water droplets with the porous substrate during processes such as coating process. Three different mechanisms co-exist in the coating process: water spreading, absorption and evaporation. Disentangling the fundamental understanding of these phenomena can therefore be crucial for achieving a higher quality of the products (e.g. a longer shelf-life of the tablets) and for controlling the efficiency of the process.
.
The pharmaceutical tablet is well-known as being amongst the most convenient ways of delivering active pharmaceutical ingredients (APIs) to the recipient. The development of APIs is the main focus of industrial and academic research in terms of medicinal efficacy. The tableting and delivery functionality of a formulation, however, is dominated by two main components, the excipient, which is present in by far the greater quantity, and the API, which is designed to have the specific in-vivo activity
Pan Coating
Pan coating is one of the first and most used methods for applying a film coating onto a tablet. The process consists of a batch of tablets deposited onto a rotating bed. The spray covers the tablets with a coating solution. Heated air evaporates the solvent leaving a solid film on the surface of the tablets. Residual moisture in the porous tablet can cause swelling, which affects coating morphology and may accelerate drug degradation and affect the dissolution behaviour. Hence, understanding and predicting the spreading, absorption and evaporation mechanisms are crucial for reducing moisture content in the tablet. The three different mechanisms of water transport and removal occur at different time scales. A liquid droplet impacting on a porous surface induces a spreading on the surface and absorption inside the porous material due to capillary forces. In particular, droplet spreading is a fast process of the order of milliseconds while evaporation takes place in the range between a few seconds and minutes. The dynamics of spreading and absorption depend on different physical parameters. The spreading rate depends on the properties of the liquid (density, viscosity and surface tension), initial droplet geometry and impact velocity, wettability and surface roughness. Absorption is controlled by the properties of the liquid and the porous substrate (porosity, pore size, wettability). During the process of coating, a droplet can rebound, splash, or simply deposit when impacting the tablet, depending on the velocity, size and viscosity of the atomized droplet. when the impact velocity is low and the spreading and absorption dynamics are strongly related to the Pharmaceutical tablet surface roughness, and, more generally, to its microstructure. The liquid droplet impact on porous surfaces has been studied previously for several different applications. For instance, inkjet printing and spray coating of paper, spray cooling, environmental applications and spray coating of tablets.
.
The use of medicines at home
sometimes, in primary care patients are requested to schedule and manage their own complex therapies independently. However, many people who take medicine sever day receive help from family and friends. Even if the level of support provided is fairly minimal, never the less it is important. Taking medicines or helping others with medicines can present problems and concerns for carers. Different storage places in the home, various formulations and pack sizes of formulations, variable need for regular and ‘as required’ medicines, complex or frequent dosing regimens may cause difficulties. There may be problems with remembering doses, opening packages, cutting up tablets, administering eye drops or encouraging people to take medicines. Patients and carers have views and concern about medicines related to their effectiveness, side effect profile, concomitant therapies and generic drug products. In particular any change in the routinely administered drug product could confuse and concern the older patient. In the home setting, therapist her is that the patient and/or carer could modify the treatment.

Pharmaceutical Tablet Standards
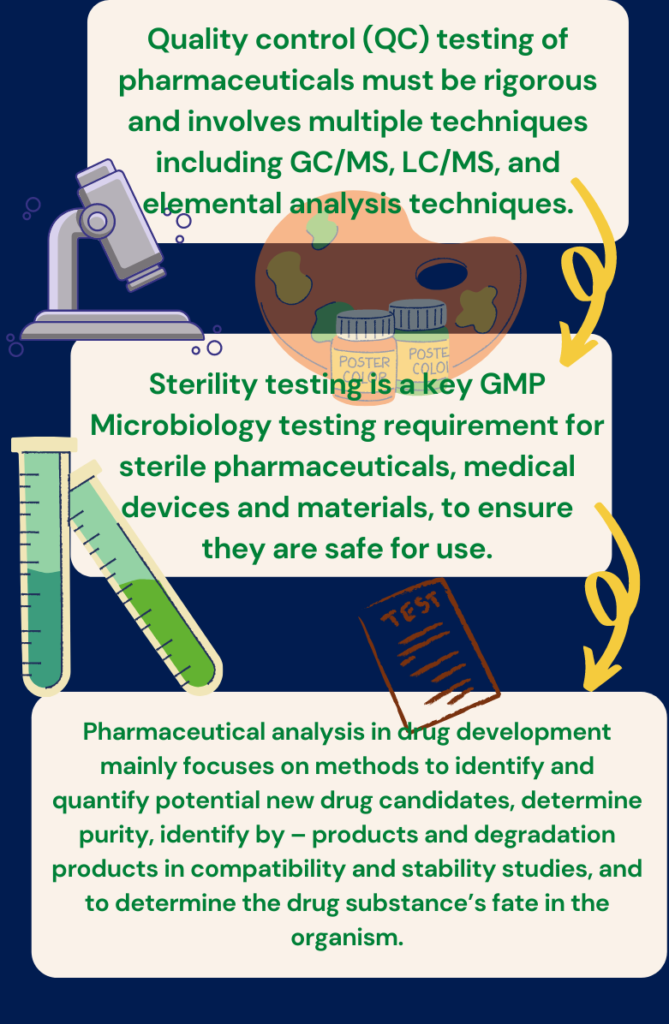
The pharmaceutical tablet must meet specific standards to claim it as a standard drug approval. Pharmaceutical industries test the Pharmaceutical tablets for maintaining their accuracy following different standard parameters such as identity, strength, quality, purity, and stability, etc. For what why, it is essential to control pharmaceutical processes regardless of the issues that may be addressed. Process control includes inspecting raw materials, controlling processes, and targeting for the finished product. That’s why it is significant to monitor the effectiveness of the process control. In connection to this, the adaptation of the production process should comply with the specification as needed, which may also include control of equipment and environment. Pharmaceutical products in the process should be checked appropriately for their identity, strength, quality, and purity and the products are approved or rejected by the quality control unit during the manufacturing process. The highlights of this review are to describe quality control testing of pharmaceutical products by using different instruments for the pharmaceutical industry, according to pharmacopeia’s
.
Pharmaceutical analysis for quality control testing in pharmaceutical industry
The evaluation of pharmaceutical raw materials and finished products for impurities and degradation products is an essential part of the process of drug development and production. Furthermore, toxicity data must be found on any drug-related impurities that represent a concentration of more than 0.1% of the active pharmaceutical ingredient(API). Conventional analysis of pharmaceutical QC and production fields has traditionally been performed by titration, Identification, Loss on drying, sulfatase, dissolution, and disintegration test by using UV-Visible, HPLC, GC, or IR detection
Quantitative analysis of pharmaceutical substances
Drugs can be either in the form of raw materials or in formulas. These can be synthesized by creating appropriate drug solutions in the solvent and measuring absorption at specific wavelengths. The Diazepam tablet can be analysed methanol by 0.5% H2SO4 at wavelength 257 nm. Qualitative analysis through spectrophotometric methods yields fast and accurate results using only small sample volumes. This fast and efficient material has become an indispensable tool in the pharmaceutical industry, thanks to its adaptability and economic value. Qualitative analysis has proven to be highly effective at many large levels of organic compounds and this helps to ensure the health and safety of the patient